Cilia regeneration in a protist
a.k.a. the fastest paper I have ever been a part of
Cover image was collected by Aidan Fenix and is a 3-D image of the microtubules in a Stentor cell. Height is represented by color.
Published as:
-
The phenomenon of ciliary coordination has garnered increasing attention in recent decades and multiple theories have been proposed to explain its occurrence in different biological systems. While hydrodynamic interactions are thought to dictate the large-scale coordinated activity of epithelial cilia for fluid transport, it is rather basal coupling that accounts for synchronous swimming gaits in model microeukaryotes such as Chlamydomonas. Unicellular ciliates present a fascinating yet understudied context in which coordination is found to persist in ciliary arrays positioned across millimetre scales on the same cell. Here, we focus on the ciliate Stentor coeruleus, chosen for its large size, complex ciliary organization, and capacity for cellular regeneration. These large protists exhibit ciliary differentiation between cortical rows of short body cilia used for swimming, and an anterior ring of longer, fused cilia called the membranellar band (MB). The oral cilia in the MB beat metachronously to produce strong feeding currents. Remarkably, upon injury, the MB can be shed and regenerated de novo. Here, we follow and track this developmental sequence in its entirety to elucidate the emergence of coordinated ciliary beating: from band formation, elongation, curling and final migration towards the cell anterior. We reveal a complex interplay between hydrodynamics and ciliary restructuring in Stentor, and highlight for the first time the importance of a ring-like topology for achieving long-range metachronism in ciliated structures.
Background
In 2018, I was a student in the MBL Physiology Course and spent two working with Wallace Marshall on Stentor, a giant complex single-celled organism that can fully regenerate itself (even after being cut in two). I collaborated with some of the other course participants (Kirsty Wan and Aidan Fenix), united by our intrigue of Stentor’s regenerative abilities. This project ended up being more than we could handle during our two week rotation at the Physiology course, so Kirsty and I went to Wallace’s lab in 2019 for two additional weeks where we collected the majority of the movies that ended up making it into the manuscript.
Introducing Stentor coeruleus, the coolest organism I’ve ever worked with
Stentor coeruleus is a crazy organism. Stentor are singled-celled protists with a complex body plan that can be up to 2 millimeter big (you can see the individual cells with your naked eye!) and are capable of regenerating themselves when injured. Even if a single cell is cut into small pieces, fragments of the cell that are as small as 0.1% of the original organism are capable of regenerating into a normal looking cell [ref]. Why doesn’t the cytoplasm leak out when the cell is injured? How does the cell know what parts of it are missing and what parts are missing and need to be regenerated? Stentor manages to completely challenge our understanding of what a single cell can even do.
A single Stentor cell healing itself and recovering parts of its cytoplasm after being massacred by a glass needle. [source]
Despite only being a single cell, Stentor has an obvious mouth. On one end of the cell, there are large (oral) cilia packed together into membranelles arranged into a ring-like structure called the membranellar band. The cilia in the membranellar band beat synchronously to form large currents, which can draw food towards the cell.
Stentor coeruleus beating the membranellar band to create a large feeding currents. 1 µm latex beads are added to the media in order to visualize the current. The movie is playing back in real time.
I found these movies of Stentor feeding absolutely mesmerizing - a single cell, composed of proteins that act on nanometer ($10^{−9}$ m) length scales, is able to coordinate the motion of its cilia which are on the order of microns ($10^{−6}$ m) long to create currents that are on the millimeter ($10^{−3}$ m) length scales. One of the other students who was also working with Wallace, Kirsty Wan, was (and still is) cilia-obsessed, so we decided to team up.
Considering that you can completely remove the membranellar band from Stentor and have the cell fully regenerate it, this gave us an avenue to understand how the cilia are able to coordinate: when during regeneration do the cilia in the membranellar band regain coordination? What can this tell us about the origins of cilia coordination in Stentor coeruleus?
Kirsty and I lacked the finesse necessary to surgically remove the membranellar band, so we utilized a curious fact about Stentor biology: when exposed to certain chemicals, including sucrose (table sugar), Stentor coeruleus will lose its old membranellar band and regrow a new one over the course of roughly 8 hours in a highly reproducible manner [ref].
A culture of Stentor coeruleus swimming around in a dish. Each of the green specks is an individual cell. [source]
A micrograph of a single Stentor cell. The anterior of the cell features a membranellar band, which terminates in a gullet or oral pouch. Cells have a macronucleus, a string of beads which contains 1000s of copies of the genome. [source]
How to re-grow a new mouth (according to Stentor coeruleus)
A short primer on how Stentor makes a new membranellar band [ref]:
The new oral cilia first appear on the side of the cell, reaching their full length after roughly 3-4 hours.
After 4-5 hours, the cilia assemble into membranelles, tightly packed cilia that act as a single unit, and the new membranellar band continues to lengthen.
After roughly 7 hours, an intracellular fiber connecting the membranelles together is regenerated and the membranellar band begins to migrate towards the mouth-end of the cell.
After 8 hours, the newly formed membranellar band is fully situated in its final position.
After being exposed to a sucrose shock and shedding its membranellar band at time t = 0 h, Stentor coeruleus will regrow a new membranellar band, shown in red. As is often the case with regeneration, this sequence is also elicited during normal development, when a cell undergoing cell division must form a new membranellar band for its daughter. Additionally, the macronucleus, shown in blue, undergoes dramatic shape changes during cell division and regeneration. Kirsty drew this during our visit to Wallace’s lab in San Francisco.
Going into this, we hypothesized that after ~7 hours the new membranellar band would be well coordinated, because:
The cilia are long enough and close enough together that hydronamic interactions between neighboring cilia could help coordinate them [ref]
The intracellular fiber is regenerated. Severing this fiber stops ciliary coordination, implying that it is necessary for the coordination of the membranellar band [ref]
In order to actually figure out when the new membranellar band becomes well coordinated again, we used two different techniques:
The individual cilia beat quickly (about 20 times a second), so we used high speed imaging (1000 frames per second) to look how well synchronized the new oral cilia are.
High speed imaging of regenerating cilia 7 hours 30 minutes after a sucrose shock. Although there is some synchronicity of the cilia, it does not persist for long. Video is 1/5th real speed
High speed imaging of the regenerating cilia from the same Stentor cell as shown on the left, 8 hours 30 minutes after a sucrose shock. At this point, the membranellar band is completely regenerated and we can see persistent synchronicity of the oral cilia. Video is 1/5th real speed.
Looking at these, it became obvious to us that our initial hypothesis was wrong: the cilia only become highly synchronous after the membranellar band is basically completely regenerated and has migrated to its final location. This meant that hydronamic interactions and the intracellular fiber are not sufficient for highly coordinated cilia. Considering that the cilia only becomes highly synchronized once it is in its final location, this led us to hypothesize that the membranellar band needs to be oriented into a circle to see highly coordinated cilia.
We wanted a way to quantitate these observations we were making. We went back and forth on precisely how to do this, at one point considering segmenting the cilia before realizing that this would be technically very difficult to do reliably for the hundreds of thousands of frames that we had collected. In the end, I developed a pipeline see how the coordination of the cilia changed over time that relied on the fact that as the cilia beat, their greyscale intensity changes.
I manually drew a line along the center of the membranellar band for each movie. Along every point on this line and for every time point, I calculated the mean intensity of a small rectangular box oriented perpendicular to the line. From this, I calculated the autocorrelation of this signal in both the spatial and temporal dimensions. To my knowledge, this was a novel analysis method of characterizing how well coordinated the cilia are.
The quantitation matched our initial observations: robust ciliary coordination only appears after the regeneration of the membranellar band is completed and in its final position.
(a) A motion heatmap was used to localize the beating cilia, e.g. to a narrow band on the surface of the organism (arrow in the direction of increasing arclength from posterior to anterior). A region of interest parallel to the ciliary band (red), was used to generate intensity kymographs. Inset: kymograph reveals local image structure and coherence.
(b) The 2D intensity autocorrelation shows increasing ciliary coordination over the course of regeneration. Sustained metachronal waves (parallel lines) only emerge once membranellar band regeneration has been almost completed. Labels indicate time post sucrose shock.
Spatial and temporal correlations of cilia
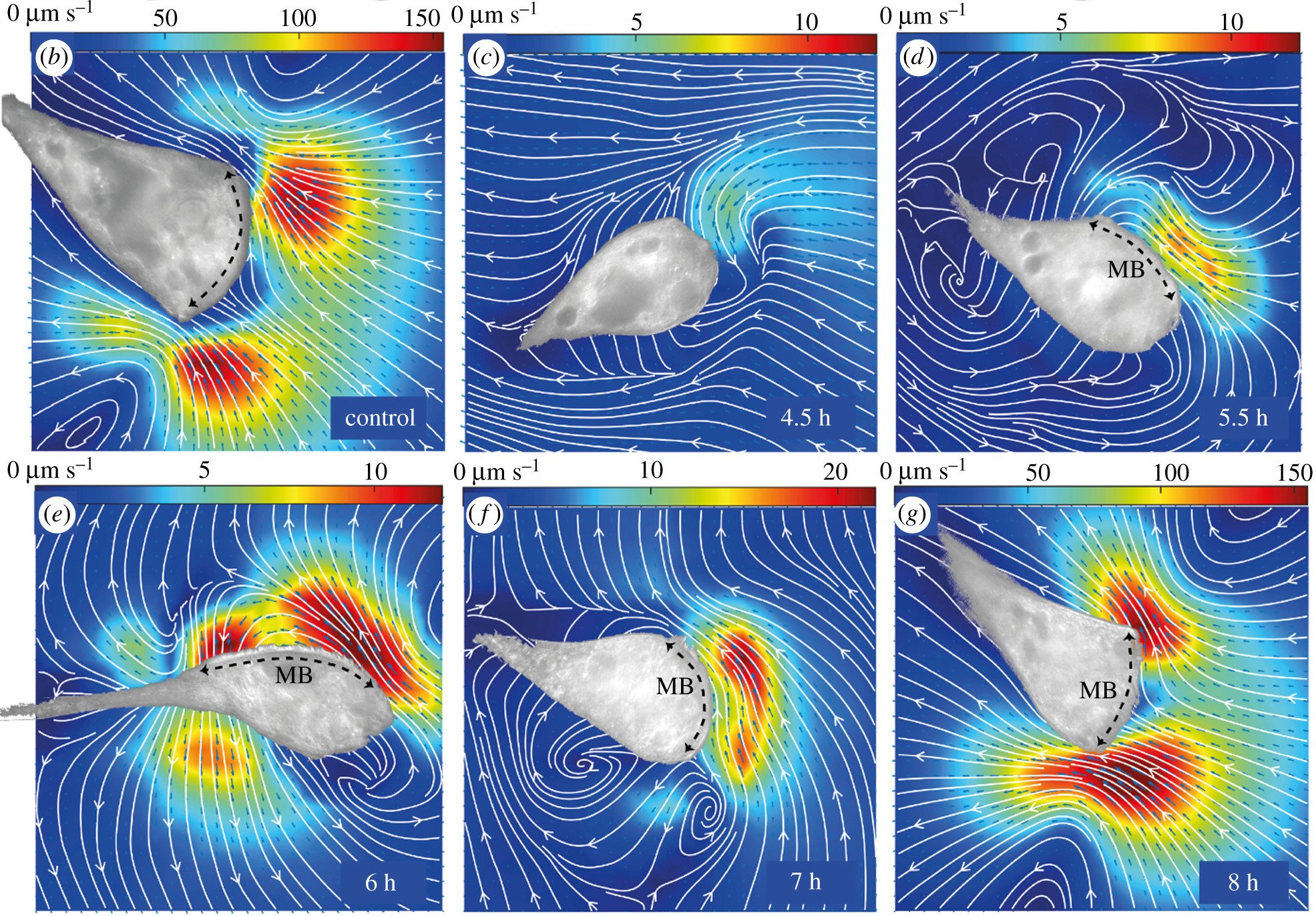
Because the ciliary coordination has a functional role (making feeding currents), we wanted to measure the flow fields that Stentor made during regeneration. We added passive tracer latex beads into the solution and Kirsty performed particle image velocimetry (PIV), which calculates the cross-correlation of small sub-images to generate flow fields.
Perhaps unsurprisingly given the cilia coordination data, what we saw is that Stentor is only capable of generating strong feeding currents when the cilia are well coordinated (note the different scale bars for the different images):
Particle image velocimetry (PIV) measurements of the extracellular flow fields associated with the regenerating MB for a control cell, and at the indicated times post sucrose shock for different regenerating individuals. (Colormaps indicate flow speed, which changes significantly during regeneration. Brightfield images of the adhered organisms have been overlaid as masks on top of the flow maps. Black arrows label the MB location—wherever it is clearly identifiable.)
This data supported what we saw with the cilia coordination data, which is that geometric orientation of the membranellar band into a circle might be necessary for the cilia to generate strong feeding currents.
-
In addition having oral cilia, Stentor also has short cilia around the cell for swimming. In order to take timelapse videos of the regenerating oral cilia, which are in a relatively narrow focal plane, we need to actively immobilize Stentor. Otherwise, they tend to roll or swim out of the focal plane.
Unlike bacteria such as Bacillus subtilis which are surrounded by a thick cell wall, Stentor is squishy and so if we try to immobilize Stentor by placing it between a glass coverslip and another rigid surface, the cell will just get flatten and attempt to swim away anyway. People in Wallace’s lab tried to solve this problem by coating a glass coverslip with Cell-Tak, a strong adhesive, and allowing the Stentor swimming around in solution ontop the glass to adhere to the coverslip. Unfortunately, this wasn’t amenable to imaging a single cell over the course of several hours (how long it takes for the membranellar band to regenerate) because the Cell-Tak is too sticky: at some point the Stentor inevitably tries hard enough to swim away that it ends up ripping itself to apart.
Luckily one of the physiology students, Aidan Fenix, had the suggestion to use polylysine instead, which is commonly used in mammalian tissue culture to more gently promote cell adherence to surfaces. By coating the glass coverslip with polylysine, we were able to create a surface that was sticky enough to immobilize Stentor on the minute-long time scale so we could acquire timelapses but would allow the Stentor to swim away uninjured if they really tried. If the Stentor dislodged themselves from the polylysine coated glass, they would re-settle a few minutes later and we could resume imaging them in their new location. We verified that the polylysine didn’t interfere with the regeneration process by timing how long it took to regrow the membranellar band with and without polylysine.
Conclusions & future directions
Although this work suggests that the geometric orientation of the membranellar band is important for ciliary coordination and flows, hydrodynamic interactions between full-length cilia and the intracellular fiber are still also necessary in Stentor coeruleus to see ciliary coordination.
Nevertheless, it would still be interesting to more directly test the hypothesis that geometric orientation is important by taking advantage of the unique regenerative capabilities of Stentor. With skilled hands, the membranellar band of one Stentor can be dissected off of one cell and grafted onto another cell and used to study ciliary and flow reorganization. In addition, previous studies have shown that it is possible to sever the oral cilia and have them regrow in place, which does not require morphological changes in the cytoskeleton; this would decouple intracellular changes from passive hydrodynamic interactions between cilia during regeneration [ref].
The strong feeding currents created by Stentor. Currents are visualized using fluorescent 1 µm latex beads. The Stentor cannot be seen but is the black hole in the center of the movie.